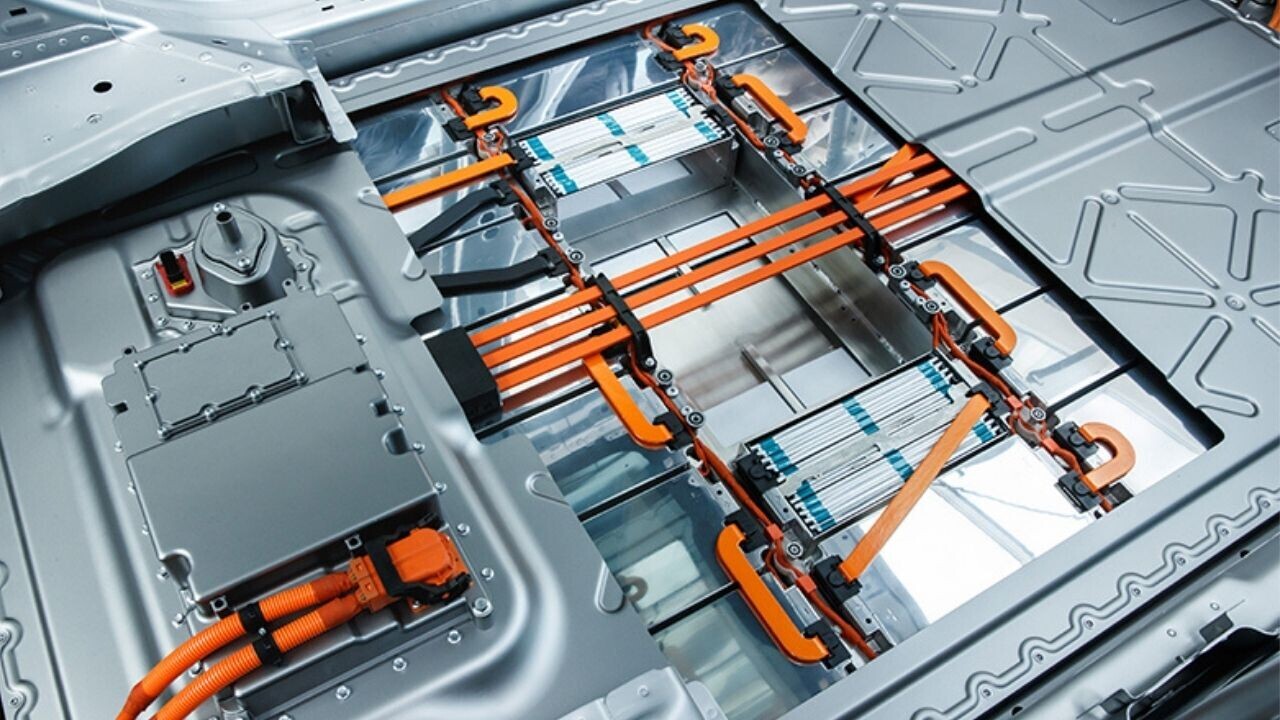
“Gigafactories” could one day be churning out millions of electric vehicle batteries in the UK. The government has already committed the country to a ban on selling new petrol- and diesel-engined cars by 2030, so it seems that electric vehicles (or EVs, as they’re often abbreviated) are likely to replace much of today’s fleet.
The carmaker Nissan has promised to beef up EV production at its Sunderland plant in north-east England, while its industrial partner is set to build an electric battery plant nearby. Meanwhile, in Cheshire, Vauxhall’s owner Stellantis has announced it will invest £100 million (US$139 million) into building electric vans and cars at its Ellesmere Port plant.
What will all these batteries look like? Most EVs today use lithium ion batteries, but these have a number of limitations. Luckily, scientists and engineers are exploring a number of ways to overcome these challenges that could help give the drive to convert cars to electricity a boost.
Lithium-ion batteries were first marketed by Sony in 1991 and have come to be the most prevalent rechargeable battery in vehicles, just as they are in mobile phones and laptops. They are more efficient and have longer lifetimes – between 15 and 20 years, about three times that of a traditional lead-acid battery. Crucially, lithium-ion batteries store more energy and are also much lighter, meaning a vehicle equipped with one uses less energy to move.
The batteries generate energy by moving charged particles called ions backwards and forwards between two electrodes. When the battery is charged, lithium ions pass from a metal oxide compound electrode to a graphite electrode. When the battery is discharged to power the car, the lithium ions go the other way, causing electrons to flow in the connected electrical circuit.
The future of EV batteries
In order to make lithium-ion batteries cheaper, scientists at Pennsylvania State University in the US are looking at lithium iron phosphate batteries, which use different electrode elements. This battery model is much cheaper and safer than the widely used lithium nickel manganese cobalt oxide batteries, and has the potential to power a car 250 miles on as little as ten minutes’ charge.
Anxiety around the range fully charged EVs can cover is also driving carmakers to develop batteries which use a solid component that separates the electrodes, rather than a liquid one. These are safer and can power EVs further than 300 miles on a single charge.
But lithium batteries have a problem. Lithium is a relatively rare element on Earth compared with most minerals in common use. As demand for batteries increases, the price of lithium will increase sharply. This has prompted geologists to search for new sources of lithium worldwide, often with their own high costs. For example, the extraction of lithium from salt flats in Chile consumes lots of water, which is in short supply there. Cobalt is also scarce compared with similar metals like iron, and ores are concentrated in the politically unstable Congo region of Africa.
One solution may be to get more use out of what we already have. With more than a million electric cars sold worldwide in 2017, a number increasing rapidly, scientists are studying how to recycle lithium on a massive scale. Some are considering whether bacteria could help them achieve this.
In future, it will be important to design batteries that can be easily disassembled, to reuse the metals they contain. Lithium is also a very reactive metal, presenting challenges for people tasked with handling it.
There are also potential alternatives to lithium. For example, sodium-ion batteries are gathering interest from EV manufacturers due to their lower cost. They work similarly to lithium-ion batteries but sodium is heavier and stores less energy.
Somewhat further into the future are multivalent batteries, where the ion that moves between electrodes has a greater charge than lithium and so delivers more than one electron each to the circuit. There are substantial challenges for scientists to overcome with these batteries, but they could potentially deliver even higher energy storage.
Building enough electric cars at a price that will make them cheaper than fossil fuelled alternatives is a major challenge. At the fore of battery research, scientists are working to solve this problem and revolutionise how we travel.
This article by Simon Cotton, Senior Lecturer in Chemistry, University of Birmingham, and republished from The Conversation under a Creative Commons license. Read the original article.
Get the TNW newsletter
Get the most important tech news in your inbox each week.





